Researchers develop a recyclable catalyst that uses CO2 to produce benzimidazoles
by Carey Sargent, EPFL, NCCR MARVEL
CO2 is a relatively unreactive molecule though and to date, the discovery of efficient catalysts to transform or reduce CO2 has been challenging. Research has nonetheless shown that so-called Frustrated Lewis pairs (FLPs)— compounds or mixtures that show “unquenched” reactivity because the two elements of the pair are kept from joining to form a classical kind of acid-base complex—can be very effective in activating such “inert” small molecules. FLPs have been used to convert CO2 to, for example, CO, methanol and methane.
Schematic illustration of CO2 transformation using SION-105
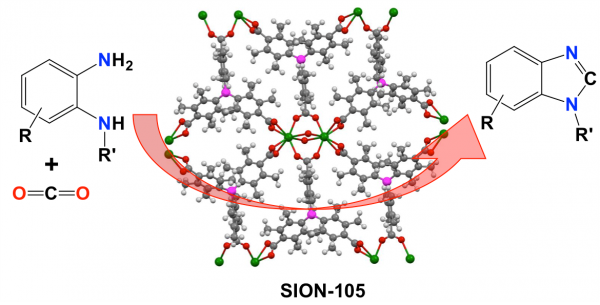
One issue though is that these FLP-based catalysts have been instable when recycled—they can be used in an initial round of chemical transformation, but then no longer can work as well. Initial efforts to get around this problem have involved the construction of FLP-like active sites in solid materials to form heterogeneous catalysts. Inspired by this approach, Dr. Kyriakos C. Stylianou and Prof. Paul Dyson from the Laboratory of Molecular Simulation and the Laboratory of Organometallic and Medicinal Chemistry, respectively, have now found a way around this, by designing and synthesizing a water-tolerant metal organic framework named SION-105 which allows for the in-situ formation of FLPs and transformation of CO2 into valuable products.
The formation of FLPs in SION-105 and the use of CO2 as a carbon source enabled the researchers to efficiently transform a variety of diamine substances into benzimidazoles, compounds used in the pharmaceutical industry, among others. They also showed that the SION-105-based catalyst can be easily recycled and reused multiple times without losing its identity and catalytic activity, underlining the advantage of SION-105 and more specifically of this MOF approach in FLP chemistry for the transformation of CO2.
Low-volume newsletters, targeted to the scientific and industrial communities.
Subscribe to our newsletter